Restricted PADRE peptide – ak(Cha)VAAWTLKAAa-Ahx-C
Universal Major Histocompatibility Complex (MHC) H2 I-Ab epitope for CD4+ T-cell recruitment and activation
SB-PEPTIDE offers I-Ab restricted PADRE peptide ak(Cha)VAAWTLKAAa-Ahx-C.
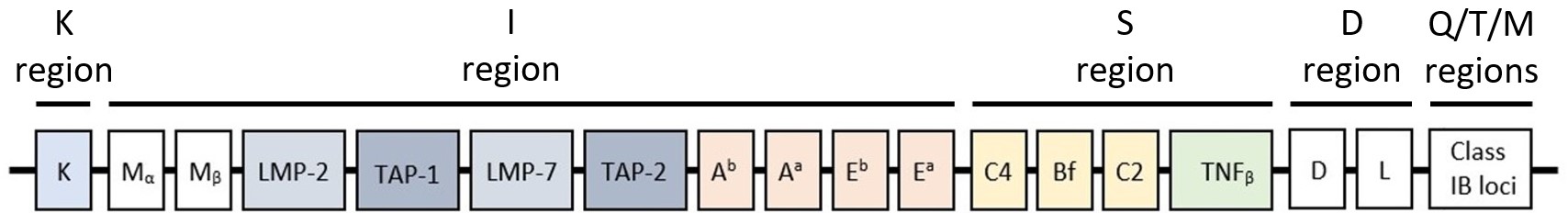
Pan DR-binding epitope (PADRE) is a universal synthetic peptide binding Major Histocompatibility Complex (MHC)-II receptors,of which H2 complex (mouse MHC),present on specific immune cells. 4 class II loci exist in the region I of the H2 complex gene (see image below),known as Aa & Ea loci (α encoded) and Ab & Eb loci (β encoded),that are similar to the DR,DP and DQ loci of the Human Leukocyte Antigen (HLA) complex.
I-Ab restricted PADRE peptide interacts specifically with mouse H2-I-Ab receptors that activate antigen specific CD4+ T-cells in the same way as PADRE peptide’s interaction with HLA-DR receptors. This feature places I-Ab restricted PADRE as promising adjuvant for immunotherapeutic vaccines against chronicle diseases such as infections,Alzheimer or cancer. Similarly to the original PADRE peptide,the restricted version also acts as a Toll-like receptor (TLR) agonist and was described as alternative carrier,such as KLH/BSA or OVA, for prophylaxis and therapeutic vaccines. An aminocaproic acid (Ahx) linker,attached to an additional C-terminal Cysteine,was added to the sequence to allow both the peptide and its loading to interact independently and freely with molecules exposed to them. This modification is used for the loading of antigenic determinants in solution in order to enhance the immune response against a specific antigen (AG).
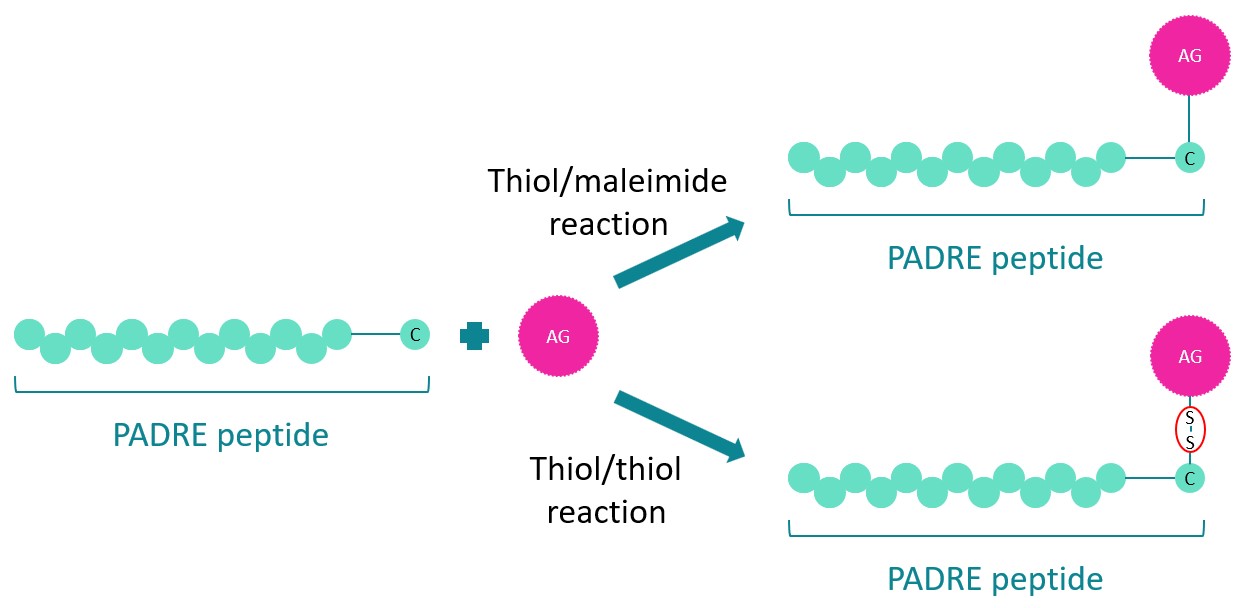
SB-PEPTIDE offers the original PADRE peptide,as well as a bioconjugation service for which the restricted PADRE peptide is eligible.
Technical specification
 |
Sequence : ak(Cha)VAAWTLKAAa-Ahx-C – Purity : > 95% |
 |
MW : 1570 g / mol (C74H124N18O17S) |
 |
For research use |
 |
Counter-Ion : TFA Salts (see option TFA removal) |
 |
Delivery format : Freeze dried in polypropylene 2mL microtubes |
 |
Other names : Restricted Pan HLA DR-binding epitope |
 |
Peptide Solubility Guideline |
 |
Bulk peptide quantities available |
Price
| Product catalog | Size | Price € HT | Price $ USD |
| SB186-1MG | 1 mg | 165 | 206 |
| SB186-5MG | 5 mg | 578 | 722 |
| SB186-50MG | 50 mg | 1980 | 2475 |
SB-PEPTIDE offers a wide range of antigens on catalog. Browse our antigen catalog to find out more.
Peptide pools are used as standards and controls to stimulate antigen-specific T-cells in functional T-cell assays. SB-PEPTIDE offers various control peptide pools including CEF control peptide pool,CMV pp65 peptide pool,Ovalbumin peptide pool.
SB-PEPTIDE can also prepare fully customized pools.
SB-PEPTIDE is a peptide manufacturing company offering a wide range of synthesis and engineering solutions.
SB-PEPTIDE offers non-conformational epitope mapping service. SB-PEPTIDE can synthesize the antigenic protein as a library of overlapped peptides (usually 15aa,5aa overlap) and perform ELISA to determine the epitope.
References
Front. Immunol. 2020 Jun 11; 11:1165. doi: https://doi.org/10.3389/fimmu.2020.01165
Peptide Vaccine Combined Adjuvants Modulate Anti-tumor Effects of Radiation in Glioblastoma Mouse Model
Glioblastoma,the most common aggressive cancer,has a poor prognosis. Among the current standard treatment strategies,radiation therapy is the most commonly recommended. However,it is often unsuccessful at completely eliminating the cancer from the brain. A combination of radiation with other treatment methods should therefore be considered. It has been reported that radiotherapy in combination with immunotherapy might show a synergistic effect; however,this still needs to be investigated. In the current study,a “branched multipeptide and peptide adjuvants [such as pan DR epitope (PADRE) and polyinosinic-polycytidylic acid—stabilized with polylysine and carboxymethylcellulose—(poly-ICLC)] ,” namely vaccine and anti-PD1,were used as components of immunotherapy to assist in the anti-tumor effects of radiotherapy against glioblastomas. With regard to experimental design,immunological characterization of GL261 cells was performed and the effects of radiation on this cell line were also evaluated. An intracranial GL261 mouse glioma model was established,and therapeutic effects were observed based on tumor size and survival time. The distribution of effector immune cells in the spleen,based on cytotoxic T lymphocyte (CTL) and natural killer (NK) cell function,was determined. The pro-inflammatory and anti-inflammatory cytokine production from re-stimulated splenocytes and single tumor cells were also evaluated. As GL261 cells demonstrated both immunological characteristics and radiation sensitivity,they were found to be promising candidates for testing this combination treatment. Combinatorial treatment with radiation,vaccine,and anti-PD1 prolonged mouse survival by delaying tumor growth. Although this combination treatment led to an increase in the functional activity of both CTLs and NK cells,as evidenced by the increased percentage of these cells in the spleen,there was a greater shift toward CTL rather than NK cell activity. Moreover,the released cytokines from re-stimulated splenocytes and single tumor cells also showed a shift toward the pro-inflammatory response. This study suggests that immunotherapy comprising a branched multipeptide plus PADRE,poly-ICLC,and anti-PD1 could potentially enhance the anti-tumor effects of radiotherapy in a glioblastoma mouse model.
Chem. Rev. 2019 Dec 05; 120,3210−3229. doi: Chem. Rev. 2020,120,3210−3229
Peptide-Based Vaccines: Current Progress and Future Challenges
Vaccines have had a profound impact on the management and prevention of infectious disease. In addition,the development of vaccines against chronic diseases has attracted considerable interest as an approach to prevent,rather than treat,conditions such as cancer,Alzheimer’s disease,and others. Subunit vaccines consist of nongenetic components of the infectious agent or disease-related epitope. In this Review,we discuss peptide-based vaccines and their potential in three therapeutic areas: infectious disease,Alzheimer’s disease,and cancer. We discuss factors that contribute to vaccine efficacy and how these parameters may potentially be modulated by design. We examine both clinically tested vaccines as well as nascent approaches and explore current challenges and potential remedies. While peptide vaccines hold substantial promise in the prevention of human disease,many obstacles remain that have hampered their clinical use; thus,continued research efforts to address these challenges are warranted.
Human Vaccines & Immunotherapeutics. 2013 Aug 08; 9:12,2566–2577. doi: http://dx.doi.org/10.4161/hv.26088
Advances in the study of HLA-restricted epitope vaccines
Vaccination is a proven strategy for protection from disease. An ideal vaccine would include antigens that elicit a safe and effective protective immune response. HLA-restricted epitope vaccines,which include T-lymphocyte epitopes restricted by HLA alleles,represent a new and promising immunization approach. In recent years,research in HLA-restricted epitope vaccines for the treatment of tumors and for the prevention of viral,bacterial,and parasite-induced infectious diseases have achieved substantial progress. Approaches for the improvement of the immunogenicity of epitope vaccines include (1) improving the accuracy of the methods used for the prediction of epitopes,(2) making use of additional HLA- restricted CD8 + T-cell epitopes,(3) the inclusion of specific CD4 + T-cell epitopes,(4) adding B-cell epitopes to the vaccine construction,(5) finding more effective adjuvants and delivery systems,(6) using immunogenic carrier proteins,and (7) using multiple proteins as epitopes sources. In this manuscript,we review recent research into HLA-restricted epitope vaccines.
