TET 830 modified/T-helper epitope from tetanus toxoid – AQYIKANSKFIGITEL
Tetanus
Tetanus is a life-threatening condition caused by Clostridium tetani bacteria. After entering the body through a break in the skin,they produce a toxin that causes convulsions and severe muscle spasms,that can be strong enough to fracture bones of the spin. Tetanus toxin inhibits neurotransmitter release by binding to peripheral neuronal synapses. The substance will then be internalized and transported to the spinal cord where it will be able to move between postsynaptic and presynaptic neurons.
For the time being,there is no cure for tetanus. Indeed,the extreme potency of the tetanospasmin toxin prevents immunity in infected people,leading to 30-40% deaths among this population. Symptoms usually start 4 to 21 days after infection,and most of the people get them after about 10 days.
Tetanus vaccine
Thanks to the widespread use of tetanus vaccines,infections are rare in many countries. However,this disease remains a threat for people without access to vaccination. Immunization against tetanus is recommended for all infants between 6 and 8,all children,and all adults.
Immunization is achieved by 3 or 4 injection of tetanus toxoid (inactivated toxin) completed by booster injections every 10 years.
Tetanus Toxoid
Tetanus toxoid is the altered form of tetanus toxin (UniProt: P04958),used in vaccines to prevent tetanus infection.
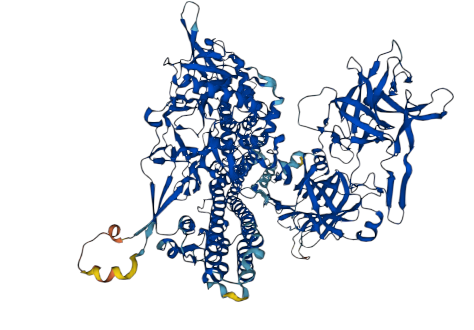
Tetanus toxin acts as a zinc endopeptidase,that cleaves the ’76-Gln-|-Phe-77′ bond within synaptobrevin-2,thereby impeding the release of neurotransmitters.
AQYIKANSKFIGITEL – Tetanus helper peptide
Among the 1315 amino acids composing tetanus toxoid,researchers identified the fragment (830-844) QYIKANSKFIGITEL (see #SB305 – TET 830 peptide) that binds to MHC class II molecules as a nonspecific vaccine adjuvant to induce and increase the helper T-cell response.
Later addition of Alanine at the N-terminal of the sequence turned the peptide into a universal human tetanus toxin T-cell epitope that can be used as helper peptide in vaccinations.
Technical specifications
 |
Sequence : AQYIKANSKFIGITEL |
 |
MW : 1796 ,07 g/mol (C83H134N20O24) |
 |
Purity : > 95% |
 |
Counter-Ion : TFA Salts (see option TFA removal) |
 |
Delivery format : Freeze dried in propylene 2mL microtubes |
 |
Peptide Solubility Guideline |
 |
Bulk peptide quantities available |
Price
| Product catalog | Size | Price € HT | Price $ USD |
| SB303-1MG | 1 mg | 165 | 206 |
References
J of Clinical Oncology. 2003 Nov 01; 21(21):4016-4026. doi: https://doi.org/10.1200/jco.2003.10.005
Clinical and Immunologic Results of a Randomized Phase II Trial of Vaccination Using Four Melanoma Peptides Either Administered in Granulocyte-Macrophage Colony-Stimulating Factor in Adjuvant or Pulsed on Dendritic Cells
Purpose: To determine clinical and immunologic responses to a multipeptide melanoma vaccine regimen, a randomized phase II trial was performed.
Patients and Methods: Twenty-six patients with advanced melanoma were randomly assigned to vaccination with a mixture of four gp100 and tyrosinase peptides restricted by HLA-A1, HLA-A2, and HLA-A3, plus a tetanus helper peptide, either in an emulsion with granulocyte-macrophage colony-stimulating factor (GM-CSF) and Montanide ISA-51 adjuvant (Seppic Inc, Fairfield, NJ), or pulsed on monocyte-derived dendritic cells (DCs). Systemic low-dose interleukin-2 (Chiron, Emeryville, CA) was given to both groups. T-lymphocyte responses were assessed, by interferon gamma ELIspot assay (Chiron, Emeryville, CA), in peripheral-blood lymphocytes (PBLs) and in a lymph node draining a vaccine site (sentinel immunized node [SIN]).
Results: In patients vaccinated with GM-CSF in adjuvant, T-cell responses to melanoma peptides were observed in 42% of PBLs and 80% of SINs, but in patients vaccinated with DCs, they were observed in only 11% and 13%, respectively. The overall immune response was greater in the GM-CSF arm (P < .02). Vitiligo developed in two of 13 patients in the GM-CSF arm but in no patients in the DC arm. Helper T-cell responses to the tetanus peptide were detected in PBLs after vaccination and correlated with T-cell reactivity to the melanoma peptides. Objective clinical responses were observed in two patients in the GM-CSF arm and one patient in the DC arm. Stable disease was observed in two patients in the GM-CSF arm and one patient in the DC arm.
Conclusion: The high frequency of cytotoxic T-lymphocyte responses and the occurrence of clinical tumor regressions support continued investigation of multipeptide vaccines administered with GM-CSF in adjuvant.
Clin Cancer Res. 2001 Oct 01;7(10):3012–3024.
Phase I Trial of a Melanoma Vaccine with gp100280–288 Peptide and Tetanus Helper Peptide in Adjuvant: Immunologic and Clinical Outcomes
A melanoma vaccine composed of HLA-A2-restricted peptide YLEPGPVTA (gp100280), with or without a modified T-helper epitope from tetanus toxoid AQYIKANSKFIGITEL, has been evaluated in a Phase I trial to assess safety and immunological response. The vaccines were administered s.c. in either of two adjuvants, Montanide ISA-51 or QS-21, to 22 patients with high-risk resected melanoma (stage IIB–IV). Local and systemic toxicities were mild and transient. We detected CTL responses to the gp100280 peptide in peripheral blood in 14% of patients. Helper T-cell responses to the tetanus helper peptide were detected in 79% of patients and had a Th1 cytokine profile. One patient with a CTL response to gp100 had a recurrence in a lymph node 2 years later; her nodes contained CD8+ cells reactive to gp100280 (0.24%), which proliferated in response to peptide. The overall survival of patients is 75% (95% confidence interval, 57–94%) at 4.7 years follow-up, which compares favorably with expected survival. Four of 14 patients who completed at least six vaccines subsequently developed metastases, all of which were solitary and surgically resectable. They remain alive and clinically free of disease at last follow-up. Data from this trial demonstrate immunogenicity of the gp100280 peptide and suggest that immune responses may persist long-term in some patients. The frequency and magnitude of the CTL response may be improved with more aggressive vaccination regimens. Although this Phase I study was not intended to evaluate clinical benefit, the excellent survival of patients on this protocol suggests the possibility of a benefit that should be assessed in future studies.
J Immunol. 1992 Jul 15;149(2):717–721. doi: https://doi.org/10.4049/jimmunol.149.2.717
Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination.
Synthetic constructs were assembled as multiple Ag peptide systems containing repetitive sequences of Plasmodium falciparum and Plasmodium berghei, the causative agents of human and murine malaria respectively, and two universal human tetanus toxin T cell epitopes 830-843 and 947-967. These constructs were tested for antibody production in mice and for their capacity to stimulate human PBL and tetanus toxin-specific T cell clones. A high antibody titer can be obtained in mice when multiple Ag peptide systems are injected in various adjuvants or in PBS alone. Furthermore, all constructs can activate PBL from every donor tested. However, a variable response was obtained when different clones specific for the two tetanus toxin universal epitopes were used. These constructs may represent possible candidates for a malaria vaccine.
Eur. J. Immunol. 1989 Dec;19:2237-2242. doi: https://doi.org/10.1002/eji.1830191209
Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells
To understand the effect of human MHC class II polymorphism on antigen recognition, we analyzed the memory T cell response to three tetanus toxin epitopes defined by three short synthetic peptides (p2, p4 and p30). We found that p2 and p30 are universally immunogenic, since they are recognized by all primed donors, irrespective of their MHC haplotypes. The analysis of specific clones indicates that both peptides are very promiscuous in their capacity to bind to class II. p30 can be recognized in association with DRw11(5), 7, 9 and with DPw2 and DPw4, while p2 can be recognized in association with DR1, DRw15(2), DRw18 (3), DR4Dw4, DRw11(5), DRw13(w6), DR7, DRw8, DR9, DRw52a and DRw52b. On the contrary, the third peptide, p4, can be recognized by only half of the donors in association with only DRw52a and DRw52c.
Analysis of truncated peptides shows that p30 contains three distinct epitopes, each recognized in association with different class II molecules. Therefore, the restriction specificity is already set at the level of the peptide-MHC complex and, in all cases, T cells discriminate p30 bound to different class II molecules. On the contrary, p2 contains only one epitope, which is recognized in association with all DR molecules. In this case we found two different restriction patterns. Some clones are monogamous, since they recognize the peptide in association with one DR allele, while others are promiscuous, since they recognize the peptide in association with several different DR molecules. Thus, in this case, the restriction specificity is also set at the level of the T cell receptor.
We suggest that both the promiscuous binding of peptides and the promiscuous recognition by T cells are dependent on the particular structure of the DR molecules, having a monomorphic α chain associated with a polymorphic β chain.
